What Is the Mole Ratio of Co2 to H2o
In other words the ratio of octane to CO2 is indeed 2 but it is also 18 so that is the correct answer. C25H52 38 O2 25 CO2 26 H2O.
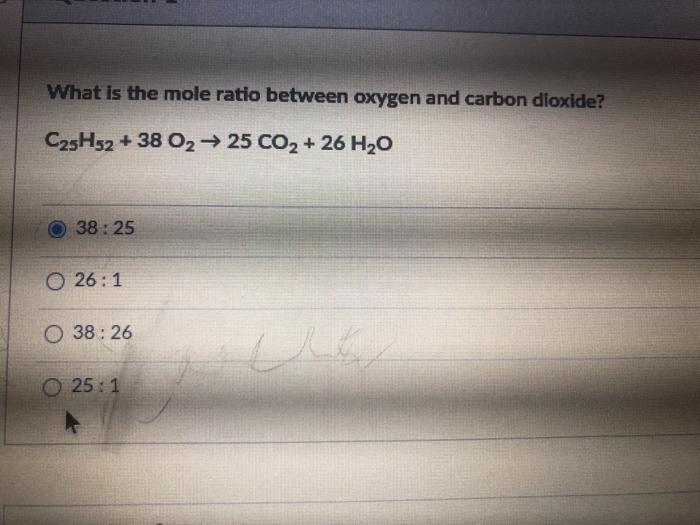
Solved What Is The Mole Ratio Between Oxygen And Carbon Chegg Com
12 CO2 molecules.
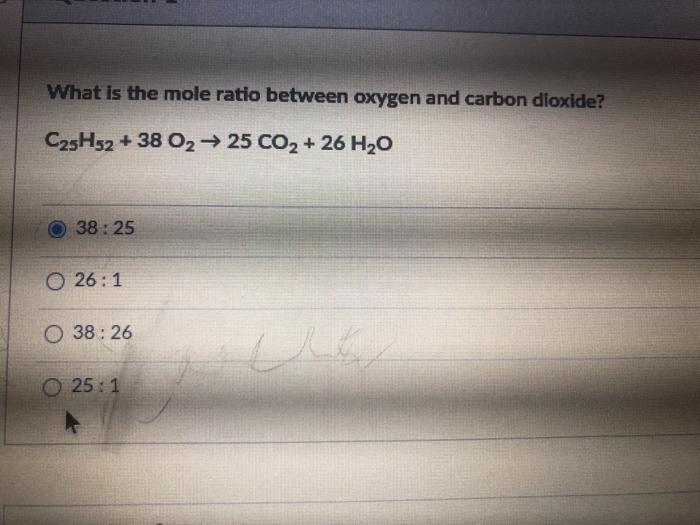
. Solve any question of Some Basic Concepts of Chemistry with-. Find the amount of HCL in mL and the grams of NaHCO3. Therefore the minimum mole ratio of O2 to C2H2 to permit complete reaction is 52.
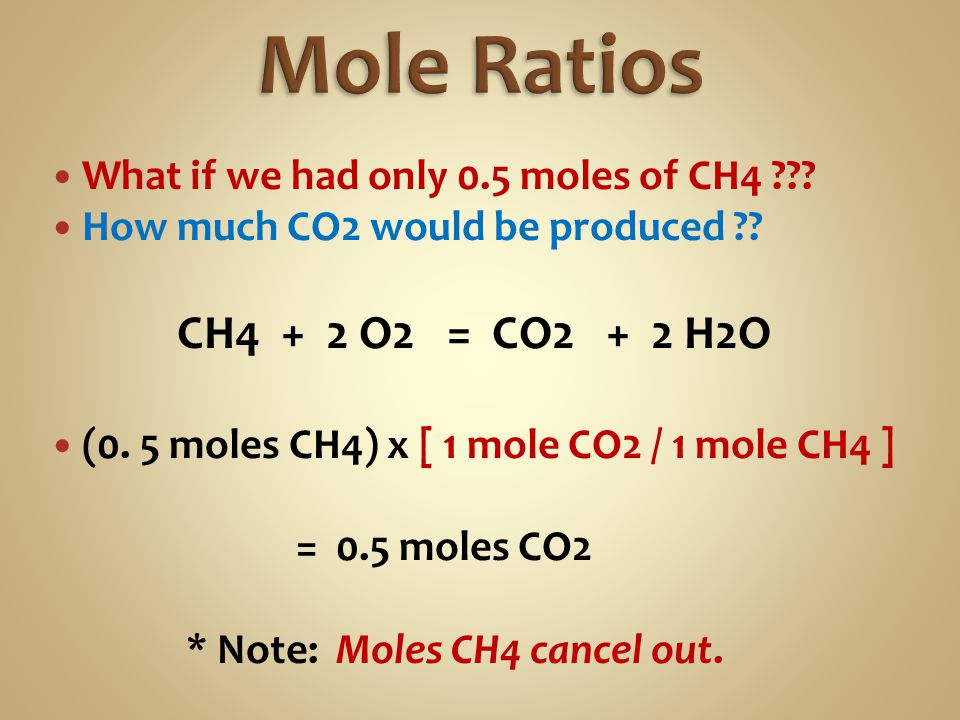
The mole concept is the concept of how many moles of a reactant reacts with how many moles of the reagent to produce how many moles of product. First things first we have to confirm if the reaction is indeed balanced. C12H22O11 12 O2 12 CO2 11 H2O.
According to the equation below what is the mole ratio of O2 to H2O. Jan 15 2014. Explain why this ratio is called the mole ratio This ratio is called the mole ratio because the reaction has happened 602x1023 times.
Since one mole is equal to 602x1023 molecules the numbers produced represent the number of moles of each reactant and product. If the density of H2Ol at this temperature. If the question had been stated in terms of grams you would have had to convert grams of H₂O to moles of H₂O then moles of H₂O to moles of O₂ as above and finally moles of O₂ to grams of O₂.
For this reaction the balanced chemical equation is. The chemical formula tells us the mole ratio. In order to produce 3 moles of carbon dioxide you need 5 moles of oxygen.
Applications of the mole ratio concept. The molar mass of carbon dioxide is 440095 gmole 2 C8H18 25 O2 --- 16 CO2 18 H20 How do I go about Chemistry 75 L of hydrogen gas at STP and zinc II nitrate was produced by reacting zinc with nitric acid. 2H 2 O2 2H 2O.
The balanced chemical reactions tells us. 1 C atom. How many molecules are there in 1 mole of co2.
It gives us the stoichiometric ratio of the reactants with the products. View more similar questions or. The molar ratio of O2 CO2 is 138 O2H2O is 1310 C4H10 CO2 is 14 and C4H10 H2O is 15.
Mg CuCl2 --. One mole of H2Og at 100 atm and 100C occupies a volume of 306 L. Determine the mass of NaHCO3 that produced the CO2 in the experiment.
1 mole of O2 2 mole H2O. It gives us the stoichiometric ratio of the reactants with the products. 12 O2 molecules.
6 mole of H2O 6 mole O2. Using the photosynthesis equation given above find the number of grams of O2 produced when 100 g of H2O are completely reacted with unlimited amount of CO2. What is the mole ratio of CO2.
CO2 1 CO2 molecule. This is what I did. 12 O2 molecules.
Zn 2HNO3 H2 Zn NO32. 1 C atom. From the reaction 38 moles of O2 produces 25 moles of CO2.
In the reaction 2 H2 O2 2 H2O what is the mole ratio of oxygen to water. Im not sure but i think so. What is the mole ratio of CO2 to C6H12O6.
What is the mole ratio of CO2. CO2 1 CO2 molecule. Mole ratio for a reaction.
The mole concept is the concept of how many moles of a reactant reacts with how many moles of the reagent to produce how many moles of product. 500 mol H₂O 1molO₂ 2molH ₂O 250 mol O₂. The equation of the reaction is given as.
These mole ratios tell you that regardless of how many moles of one reactant you have there will always be a 12 mole ratio between them. 6 mol CO2 1 mol C6H12O6 Then we set up the following ratio. Calculate the mass of zinc needed for this reaction.
A pair of unit factors can be constructed from the molecular weight or molar mass. Mole ratio for a compound. NaHCO3 HCl NaCl H2O CO2 The there are 0489 moles of CO2 and HCL is 6M.
75L H2 1 mole H2 224 L H2. How Many Moles Of Co2 Are In Octane. You can find out how much reactans will mix and how much products they will produce by using the mole ratio.
6 moles C 6 H 1 2 O 6 6 moles H 2 O is not a mole ratio of 6 C O 2 6 H 2 O C 6 H 1 2 O 6 6 O 2 1 mole C 6 H 1 2 O 6 6 moles C O 2 is the correct mole ratio. 1 C atom. The mole ratios are determined using the coefficients of the substances in the balanced chemical equation.
There are 421541024 42154 10 24 molecules in 7 moles of CO2 C O 2. Thus X is C 5 H 8 C n H 2 n 2. How many moles of O₂ are required to form 500 moles of H₂O.
We find this answer by using Avogadros number. The mole ratio between oxygen and carbon dioxide is 53 ie 5 to 3. A student used a 0358 g sample of Alka-Seltzer and the mass of CO2 was found to be 0102 g.
The molar ration of C O 2 and H 2 O produced by the combustion of one mole of hydrocarbon X is 5. The molar ratio of O2 CO2 is 138 O2H2O is 1310 C4H10 CO2 is 14 and C4H10 H2O is 15. In the equation 6 CO2 6 H2O C6H12O6 6 O2 the mole ratio of water to oxygen is.
Predict the products for the this reaction. 12 CO2 molecules. Based on this result answer the following questions.
Means the compound has 5 carbons and 8 hydrogens. A pound of sugar has a mass of 4536 grams and one mole of this sugar has a mass of 3423 grams. What Is The Simplified Mole Ratio Of Octane To Carbon Dioxide.
The ratio is given as. The chemical formula tells us the mole ratio. Similarly the reaction will always produce twice as many moles of water than of carbon.
The chemical formula tells us the mole ratio. 2 C4H10 13 O2 8 CO2 10 H2O. When one mole of H2Og is condensed to one mole of H2Ol at 100 atm and 100C 4066 kJ of heat is released.
In order to determine the mole ratio we need to begin with a balanced chemical equation. I think its c. 12 O2 molecules.
The actual ratio present 740240 or about. CO2 1 CO2 molecule.

Solved 2 Consider The Following Chemical Reaction 16 Co2 Chegg Com

Question Video Deducing The Molar Ratio Of Reactants From A Balanced Reaction Equation Nagwa
Comments
Post a Comment